Fresenius Kabi’s experience plays a significant role in why we are BioSpecialized®
Our products and pipeline
Marketed products
Our FDA approved biosimilars that ultimately provide the ability to help more patients.
Adalimumab-aacf

Our pipeline
Our pipeline
Product
Stage
rituximab*
On-Body Injector*
Filed for Approval
ustekinumab*
denosumab*
denosumab*
Multiple candidates*
*Products pending FDA Approval. The safety or effectiveness of the products has not been established.
Advanced science, manufacturing and support for biosimilars

Manufacturing and supply network

Oncology experience

Immunology expertise

Our U.S. Presence
Our Manufacturing and supply network

Our Manufacturing and Supply Network
Made up of 90 science, manufacturing and R&D centers around the globe, Fresenius Kabi is committed to making drug development more efficient. We use leading manufacturing technologies and innovative processes to replace slow and expensive legacy methods. Our extensive experimentation, analytical characterization and computational simulations ensure high-quality products from batch to commercial scale.
Our five-step process for biosimilar development
Cell Line Development
Upstream Process Development
Downstream Process Development
Analytics
In Vitro Nonclinical Pharmacology

Oncology Experience
At Fresenius Kabi, we have one of the most comprehensive, non-branded oncology portfolios in the industry and have been supplying the U.S. with oncology medicines for more than a quarter of a century.
60+
460+
220+
14,000+
20+
A leading manufacturer
The role of biosimilars in oncology
By 2030, the total spending for cancer care in the U.S. is projected to be around $246 billion. As cancer care spending continues to grow, so does patient cost-sharing.1 A 2017 report found that the average per-patient out-of-pocket costs for the first year following a cancer diagnosis were between $3,600 and $5,500 depending on cancer type, treatment required and insurance coverage.2
In their 2018 statement on biosimilars, the American Society of Clinical Oncology (ASCO) acknowledged that biosimilars will play an important role in the future care of patients with cancer and will improve access to medicines.3 The growing availability of oncology biosimilars could help to provide more treatment options, increase access to life-saving medicines and potentially lower treatment costs for both patients and payers.4
As of May 2022, there are 36 FDA-approved biosimilar products in the U.S., many of which are utilized in oncology or supportive care for patients with cancer.5,6 With increased experience and adoption of oncology biosimilar products, the substantial savings versus the reference products will also continue to increase.7
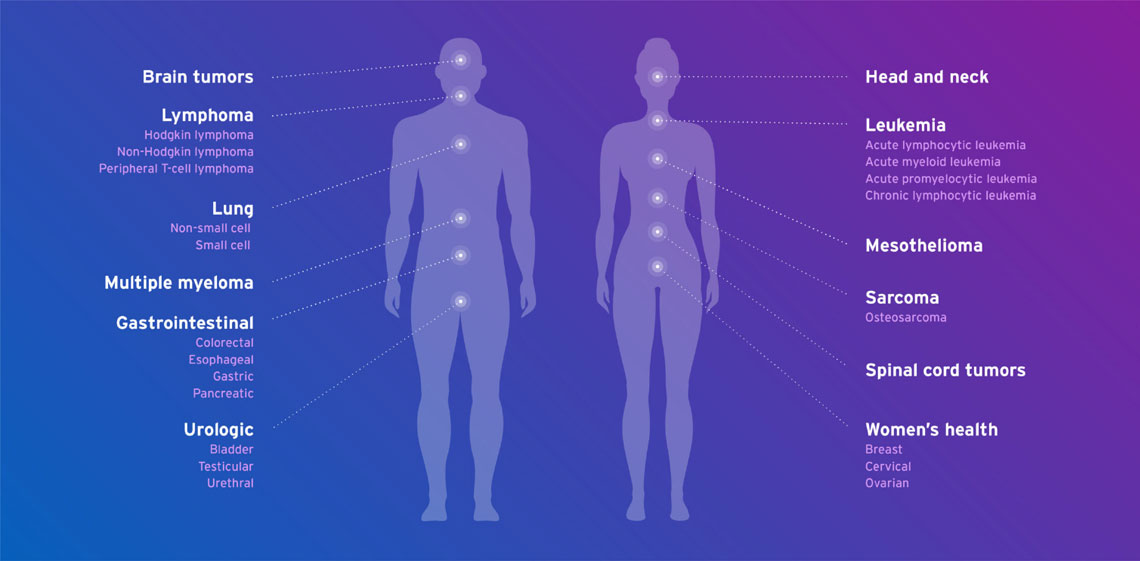
Fresenius Kabi oncology products are used in many different cancer types including:
* 6 products at #1 market share & 6 products at #2 market share. Source: IQVIA;
** Fresenius Kabi oncology products validated against chemotherapy regimen list from National Comprehensive Cancer Network (accessed 4/2020 from nccn.org)
- Mariotto AB, Enewold L, Zhao JX, Zeruto CA, Yabroff KR. Medical Care Costs Associated with Cancer Survivorship in the United States. Cancer Epidemiol Biomarkers Prev. 2020;29(7):1304-12.
- Dieguez G, et al. https://www.milliman.com/en/insight/2017/a-multi-year-look-at-the-cost-burden-of-cancer-care. Published 2017. Accessed May 5, 2021.
- Lyman GH, et al. American society of clinical oncology statement: Biosimilars in oncology. J Clin Oncol. 2018;36(12):1260-1265.
- FDA. https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/biosimilars. Published 2020. Accessed May 5, 2021.
- FDA. https://www.fda.gov/drugs/biosimilars/biosimilar-product-information Published 2020. Accessed March 18, 2022.
- Lyman GH. How biosimilars will impact costs and care in oncology. Clin Adv Hematol Oncol. 2019;17(10):544-547.
- Tilleul PR, et al. Introduction of biosimilar pegfilgrastim in France: Economic analysis of switching from originator [published online ahead of print 2020 Oct 6]. J Oncol Pharm Pract. 2020:1078155220962208.
Immunology Expertise

Immunology Expertise
Providing affordable and high-quality health care to patients coping with chronic diseases is an integral part of Fresenius Kabi’s purpose, and this is demonstrated by our long-term commitment toward developing a comprehensive portfolio of immunology biosimilars. Our biosimilars are produced in an established European facility with over 20 years’ experience in manufacturing biologics.1
35+
4+ Years
Parenteral nutrition support
Small molecules
In immunology, our pipeline includes biosimilars of adalimumab (an anti TNF alpha monoclonal antibody which is used to treat many autoimmune diseases), tocilizumab and others. In the field of biosimilars, Fresenius Kabi is continuously committed to developing new medicines in these important therapeutic areas; we have multiple candidates in various development phases, in both the autoimmune and oncology therapeutic areas.
*Full product descriptions and safety information can be found at products.fresenius-kabi.us.
- https://www.merckgroup.com/content/dam/web/corporate/non-images/country-specifics/switzerland/who-we-are-merckswitzerland-FR.pdf, retrieved 04/2022
- Data on file 4/1/22 calculation includes all ILEs approved in the U.S.
Our U.S. Presence

Our U.S. Presence
- 2021 Data on file
Getting affordable medicines to your patients
Our biosimilars history
2012
2017
2019-2021
Now
Committed to ongoing biosimilar support and guidance
Fresenius Kabi puts patients first, helping health care professionals optimize patient care through education and support programs provided to patients and families/caregivers.
Fresenius Kabi has team members in the U.S. and around the world to help physicians and patients. We provide educational support and clinical data related to Fresenius Kabi biosimilars. Through the KabiCare Patient Support Program, patients, caregivers and health care providers have access to multiple layers of support and resources throughout the treatment journey.

Fresenius Kabi at a glance
41,000
A global team of nearly 41,000 dedicated team members
90
Operating more than 90 R&D centers and manufacturing facilities worldwide
150
Serving customers in more than 150 countries
Shaping the future of health care



Operation and management of hospitals and other sites of care.

Equipment and services for dialysis and renal care.

Planning, construction, and management of care facilities.


Operation and management of hospitals and other sites of care.

Equipment and services for dialysis and renal care.

Planning, construction, and management of care facilities.
- Fresenius. https://www.fresenius.com/Group-Overview. Published 2021. Accessed June 1, 2021.
- Source: https://www.fresenius.com/financial-results